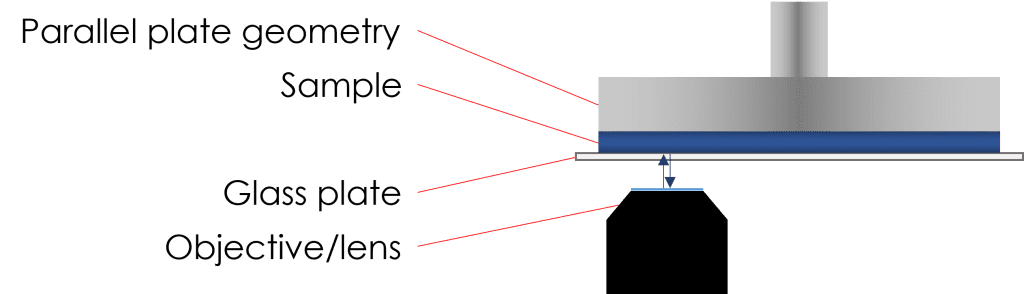
Rheo-microscopy combines the fields of rheology and microscopy into a unique, powerful tool in which both parts complement the other. It uses a traditional rheometer with a microscope mounted underneath a transparent base plate that allows the user to see visually how samples behave under shear, whilst concurrently collecting rheological data.
This includes things such as:
- Crystallisation and melt-profiles
- Dissolution
- Structural destruction/recovery during/following shear
- Agglomeration
A material’s microstructure changes upon application of shear; whilst these changes can be characterised by performing rheological tests (looking for yield stresses, non-Newtonian behaviour, viscosity, etc.), to understand and attribute the behaviour of materials under flow requires visual information, which can be captured by microscopy.
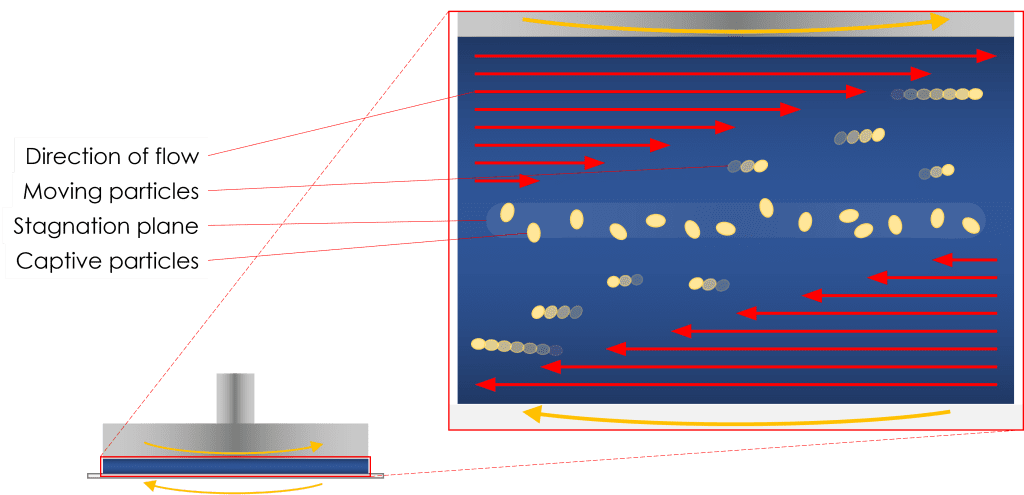
Materials under flow, by nature, can be complicated to visualise at high shear rates due to features of interest (droplets, particles, etc.) passing swiftly over the objective. To combat this, by rotating the transparent base plate in the opposite direction to that of the upper geometry, a stagnation plane can be achieved. Whilst the sample is under shear, the fluid at the stagnation plane is stationary with respect to the camera. This allows for the features of interest aforementioned to be kept in the field of view whilst under shear.
Temperature has a great influence over the flow characteristics of most substances. Melting and cooling profiles can be captured by a rheometer, tracked by changes in modulus dominance and viscosity. When paired with a microscope it can be seen, for example, that a molten wax or petroleum jelly when cooled outwaxes and crystallises, leading to an increase in viscosity. Rheo‑microscopy can be used to visualise melt profiles of a wide range of materials such as lipids, fats, waxes, gels, even cheese when stained with a dye!
Contact us for more information
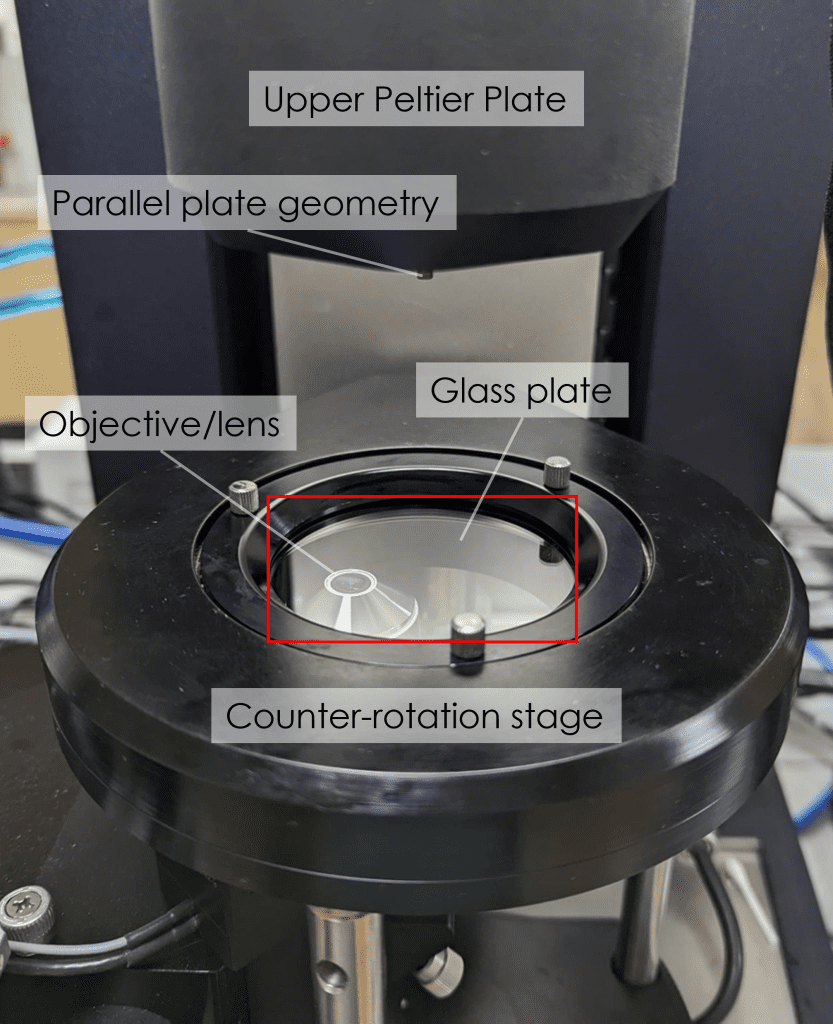
Our TA Instruments HR20 research rheometer is fitted with the MMA (Modular Microscope Accessory). Also fitted with an Upper Peltier Plate, it is capable of controlling the temperature of loaded samples between -20 °C and 100 °C. The counter-rotation stage allows for capturing the stagnation plane. We have two beam splitter blocks: the 50/50 beam splitter is used for brightfield applications and the dichroic beam splitter allows for fluorescence microscopy. A variable polariser is attached to the camera for selective illumination. An x-y-z micrometre positioning system allows for the field of view of the microscope to be set anywhere within the sample. Precise depth-profiling is achieved by a Piezo scanning system, which can adjust the focal plane over a 100 μm range. Images can be captured up to a rate of 90 fps.
