 The performance of dry powder inhalers (DPIs) are influenced by both the design of the inhaler and the inherent handling behaviour of the powder itself. Flowability, aeration and permeability of the powder are all important when investigating how the powder behaves in situations from handling within the processing line to delivery of API to the lung. Achieving ‘good flowability’ is not always as simple as it sounds, and matching testing procedures to real life situations is key to generating useful data.
The performance of dry powder inhalers (DPIs) are influenced by both the design of the inhaler and the inherent handling behaviour of the powder itself. Flowability, aeration and permeability of the powder are all important when investigating how the powder behaves in situations from handling within the processing line to delivery of API to the lung. Achieving ‘good flowability’ is not always as simple as it sounds, and matching testing procedures to real life situations is key to generating useful data.
There are multiple challenges when working with dry powder inhaler formulations. A light ‘floaty’ or ‘dusty’ powder may be excellent for inhalation but frustratingly difficult to meter a dose or contain during processing, creating some interesting challenges for engineers! Understanding the behaviour of the powder under a variety of different situations is key to optimisation – benchmarking and comparison of different powders can allow for improvements to existing products, easier and more optimal processing, and aid in pharmaceutical generic to reference submissions.
At the Centre for Industrial Rheology we work closely with scientists, formulators, and engineers to understand industrial challenges and suggest process relevant measurements. We help people with excipient comparisons, processing challenges and competitor benchmarking.
If you would like to discuss characterisation of the powder of your DPI, please feel welcome to contact us.
Flowability and Aeration – A starting point for dry powder inhalers
One of the biggest challenges in designing a dry powder inhaler is in accommodating for variable handling behaviour seen for different powder formulations in a specific environment or set of circumstances. In the same way a spray bottle is a poor dispensing method for honey, the design choices of the inhaler will vastly influence the observed performance of the powder API-excipient system. It makes sense then to look at how the powder bulk will behave in a set of controlled tests to predict what it will do. We can simulate deformation of the powder bulk in a variety of different ways.
Basic Flow Energy and Specific Energy – Baseline measurement of flowability
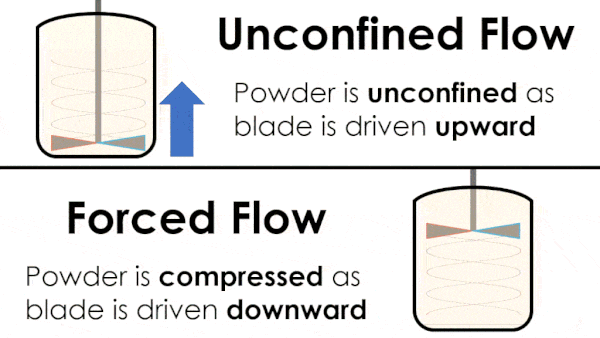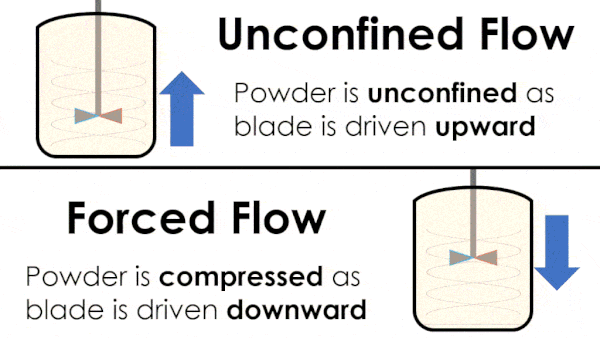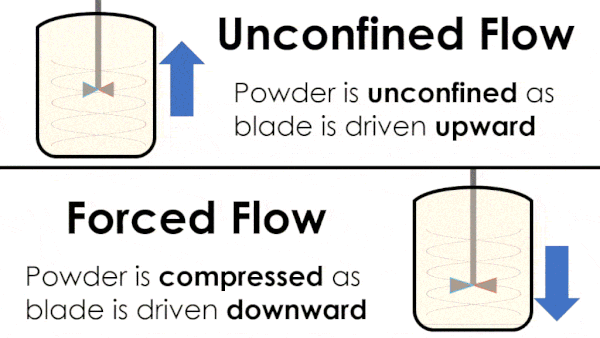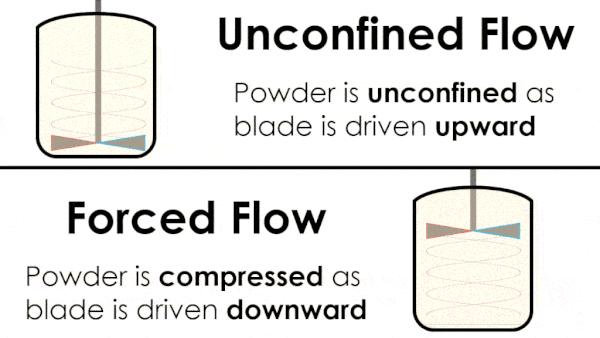 Basic flow energy measures the effort required to drive a twisted blade downwards through a conditioned column of powder. This provides an empirical measure of the powder’s handling characteristics. The test simulates ‘forced or confined flow’ situations, whereby the blade is driven downards into powder confinded by its vessel. In a ‘specific energy’ test the blade is run upwards through the conditioned column of powder.
Basic flow energy measures the effort required to drive a twisted blade downwards through a conditioned column of powder. This provides an empirical measure of the powder’s handling characteristics. The test simulates ‘forced or confined flow’ situations, whereby the blade is driven downards into powder confinded by its vessel. In a ‘specific energy’ test the blade is run upwards through the conditioned column of powder.
Aeration – Measuring the impact of aeration
For many dry powder inhalers, it is down to the patient to deliver the driving force which de-agglomerates and aerosolises the powder. It is vital to deliver a controlled amount of finely dispersed powder into the lungs for clinical efficacy, as well as for the ease and comfort of the patient. In an aerated flow test, a steady stream of air is passed through a column of powder as basic flow energy is measured. This is then compared to the basic flow energy of the powder without aeration.
Shear Cell Testing – Flow function and cohesivity
Shear cell testing is a frequently used method for generating the flow function coefficient of a powder – the ratio between the failure strength of the powder versus consolidating stress. This gives an indication of the cohesivity of the powder and helps understand general flowability. It can be very useful in process design when coupled with additional metrics such as wall friction and bulk density measurements.
Permeability – Minding the gap between powder particles
The permeability of a powder is vital to the performance of a dry powder inhaler. Permeability is indicated by the pressure drop from a steady flow of air passed through a column of powder and is a very useful metric for investigating situations involving trapped air in powder bulk. A high permeability means air flows more easily through the powder bulk.
The driving force propelling powder from the inhaler to the lungs comes from the inhalation of air by the patient. For DPIs, excipients are specifically selected to behave as microscopic sails, helping to deliver APIs into the lungs. Since DPIs are frequently prescribed for respiratory diseases, it is likely some patients will suffer from reduced lung function and may have difficulty in generating high air flow during an inhalation event.
If the powder is too permeable, the precious driving force provided by the inhaled air is wasted. This could result in a powder that is insufficiently dispersed or fails to reach its intended target. More cohesive powders may be preferable in specific inhaler designs since they create better ‘sails’ for catching inhaled air at the cost of being more difficult to de-agglomerate.
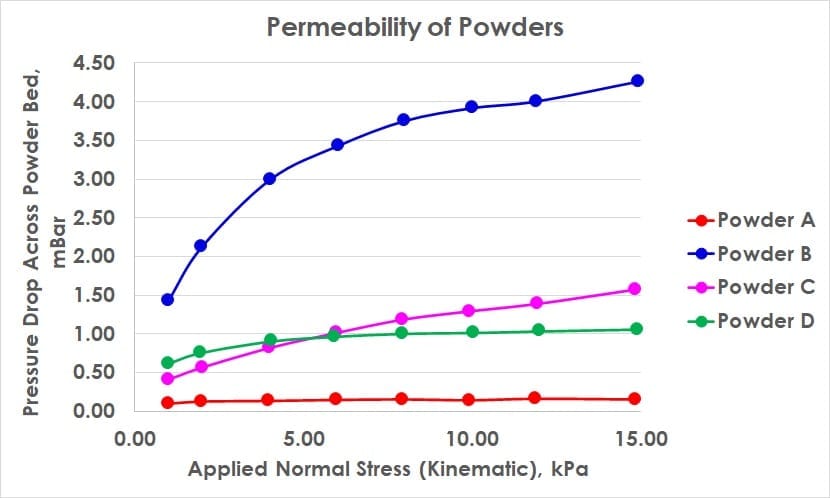
Particle size, shape, density, cohesion, friability and prior conditioning all contribute to the permeability of a powder mass. In a permeability test, the fundamental principle is to investigate whether the organisation of powder particles inhibit airflow, and to what extent compression affects this. Compression can force powder particles into closer proximity and into positions which obstruct routes for air to flow through. In the graph above, powder A is highly permeable and largely insensitive to compression suggesting the powder forms a natrually porous state where contact/proximity between particles is already at a maximum for these stress ranges. Powder B on the other hand is far less permeable and more sensitive to compression.
Powder Stability: Attrition and Segregation
 Assessing how your powder changes over time is a vital part of creating an excellent dry powder inhaler. Numerous factors can play a part in changing a powder over time. Particle attrition, consolidation, segregation, and caking can all play a part. The degree and rate at which these occur can make a tremendous difference to a powder. A dry powder inhaler fresh from processing stored in a cool, dry cupboard will be expected to behave the same as one kept in a warm, humid handbag and carried around for a month. It is vital that a dry powder inhaler resists these changes and remains stable over time, even against a wide range of environmental factors such as humidity, temperature, and physical attrition.
Assessing how your powder changes over time is a vital part of creating an excellent dry powder inhaler. Numerous factors can play a part in changing a powder over time. Particle attrition, consolidation, segregation, and caking can all play a part. The degree and rate at which these occur can make a tremendous difference to a powder. A dry powder inhaler fresh from processing stored in a cool, dry cupboard will be expected to behave the same as one kept in a warm, humid handbag and carried around for a month. It is vital that a dry powder inhaler resists these changes and remains stable over time, even against a wide range of environmental factors such as humidity, temperature, and physical attrition.
Investigating the stability, flowability and permeability of a powder is vital to assessing its performance in a dry powder inhaler. From processing to storage to inhalation, an inhaler powder can experience a huge range of environmental variances in humidity, temperature and physical circumstances. Yet, despite these factors, it is vital that a dry powder inhaler continues to perform as expected, from the first puff to the last.
